State Council vows severe punishment in vaccine case, building of institutional safeguards

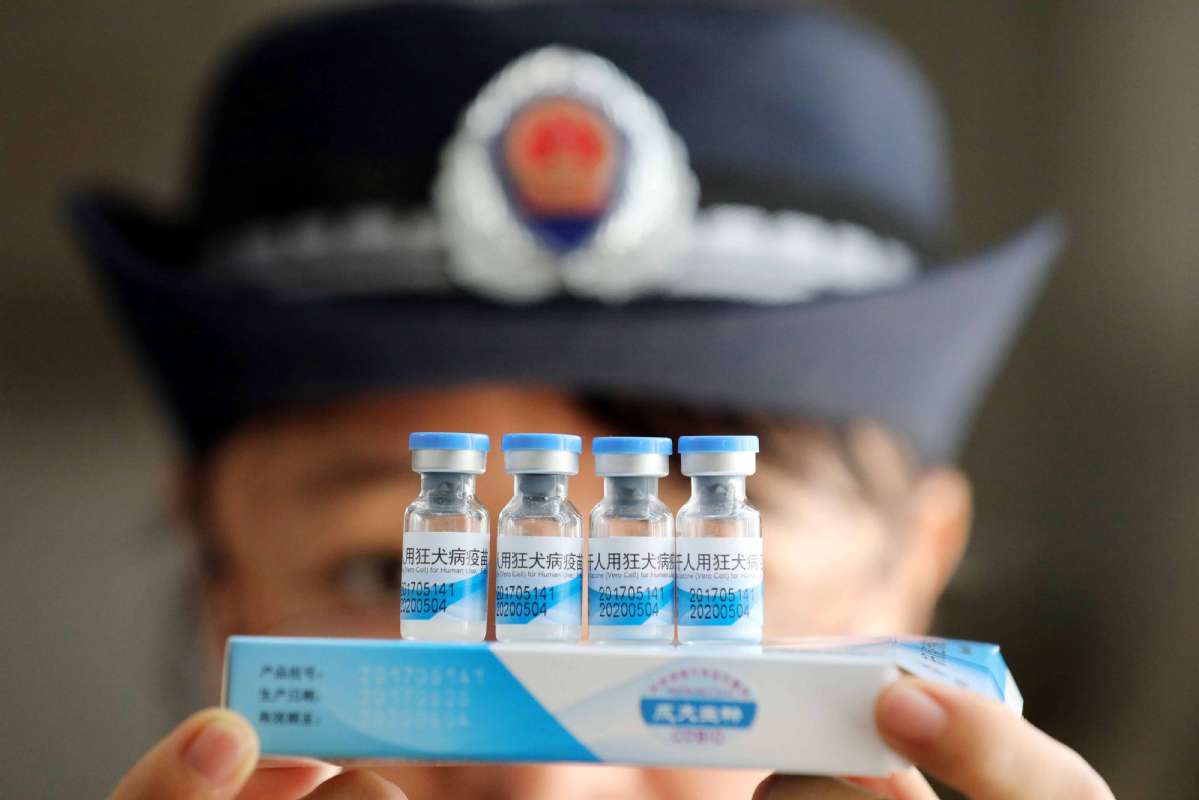
Changsheng Life Sciences Ltd, a company from Changchun city, Jilin province, held responsible for illegally producing substandard rabies vaccines for human use will receive severe punishment, and a long-term institutional mechanism for safeguarding drug safety will be established in the country, said the State Council's executive meeting on Monday.
The meeting, chaired by Premier Li Keqiang, heard the report on the case submitted by the State Council's investigation team.
The Monday meeting urged related departments to work with local authorities to recall and destroy each and every dose of the vaccine.
The meeting also called for government supervision of recall of faulty vaccines that may have been exported by the company in question and for timely briefings made to the World Health Organization.
At the same time, thorough checks will be carried out on all the other vaccines produced by the company and any substandard cases uncovered will be dealt with immediately.
"The CPC central leadership and the State Council have placed high priority on tackling this case and so far the investigation has moved forward steadily," Li said. "Given the heinous nature of this incident, tough punishment must be meted out to the culprits, and a comprehensive plan be drawn up to deal with its aftermath."
So far, the probe has found evidence that the company engaged in suspected criminal activities by reportedly violating approved production process, alleged falsification of production and inspection records, and reported destruction of evidence, severely violating national drug standards and regulations.
The Monday meeting called for expeditious steps to impose stronger punishment and heavier fines on the company and those held accountable, while State Council's investigation team will continue to work on the case. Those suspected of breaking the law will be handed over to judicial authorities for prosecution. Those found guilty of serious breach will receive jail sentences, and will be driven out of the pharmaceutical business for life.
At the same time, the government will speed up its sweeping inspections and checks on all 46 vaccine producers across China. The results will be made public in a timely fashion.
The meeting called for a thorough investigation on possible misconduct in the regulation and supervision. Any dereliction of duty will be investigated and once established, severely punished. There will be zero tolerance for law-breaking activities such as bribe-taking and illegal transfer of benefits.
"Possible criminal activities of counterfeiting and administrative dereliction of duty will be thoroughly investigated and punished with the full rigor of the law. We must make this amply clear to our people and to the international community," Li said at the meeting.
The Monday meeting also urged related government agencies to move quickly in formulating a detailed regulatory plan that covers the entire process of vaccine R&D, production, distribution and use to set up institutional safeguards for drug safety
"As part of efforts to handle this incident, we must carry out sweeping checks on the vaccine business and indeed the entire drug industry, and put together a long-term mechanism for drug safety by drawing on advanced international practice," Li said.
He instructed that detailed and sound plans be worked out to deal with problems arising from the incident.
- Ultra-cheap dress blind boxes spark health, quality concerns
- Chinese researchers find new treatment path for high-risk breast cancer
- China cracks down on organized crime involving minors
- Two Taiwan suspects wanted in mainland smuggling case
- Lhasa promotes initiative to foster a skilled workforce
- Beijing makes it easier for families to buy property